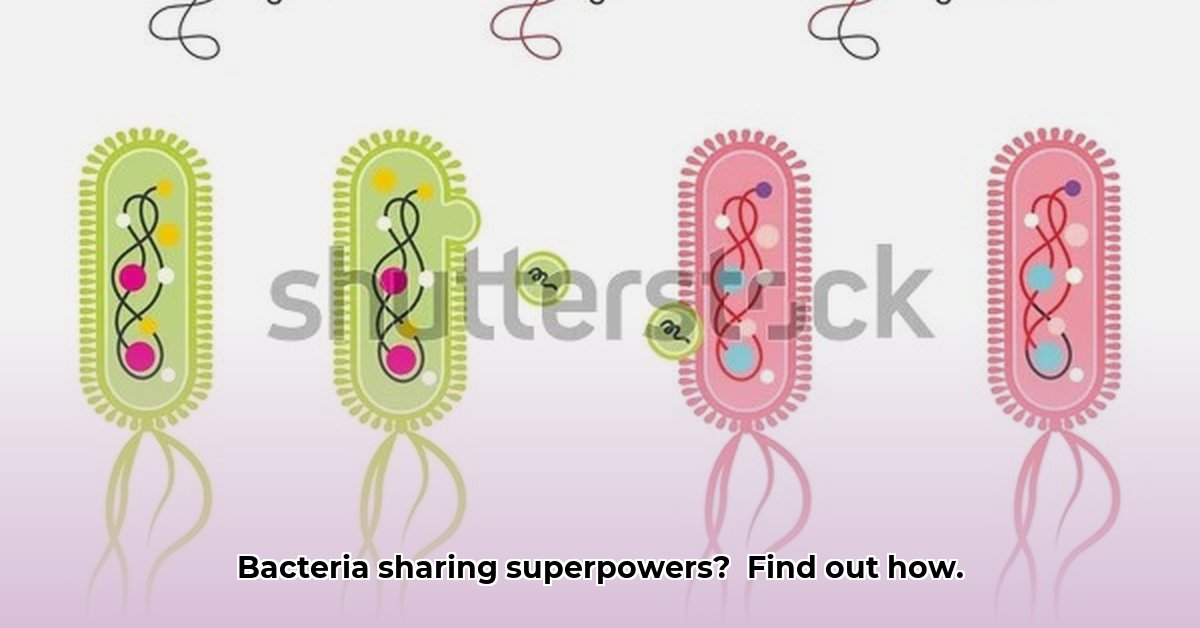
Have you ever wondered how bacteria develop superpowers? It’s not from a lab accident or rigorous training! Bacteria swap genetic material, a process called horizontal gene transfer (HGT). It’s like a microscopic internet, sharing genes that can make them stronger, more resistant to antibiotics, or even able to exploit new environments. Understanding how this happens and what we can do about it is crucial for tackling antibiotic resistance and harnessing the power of bacterial genetics.
The Gene Exchange Market: How Bacteria Trade Traits
Think of a bustling marketplace, but instead of physical goods, the currency is genes. That’s essentially what horizontal gene transfer (HGT) is. Bacteria don’t just inherit traits vertically from their “parents;” they frequently acquire new abilities by swapping genetic material with their neighbors, even distantly related ones. This accelerates bacterial evolution and has a profound impact on everything from antibiotic resistance to the health of our planet. How does this exchange work, and what are the implications?
Three Main Ways Genes Get Around: Unveiling the Mechanisms
Bacteria have evolved several ingenious strategies for sharing genetic information:
-
Transformation: Some bacteria can scavenge DNA fragments floating in their environment, often released from dead bacteria. If they encounter a useful gene, like a recipe for antibiotic resistance or a new metabolic pathway, they can incorporate it directly into their own DNA.
-
Transduction: Viruses that infect bacteria, called bacteriophages (or phages for short), sometimes act as unintentional couriers. When a phage infects a bacterium, it may accidentally package up a piece of the bacterium’s DNA along with its own. If this phage then infects another bacterium, it injects this DNA fragment, potentially introducing new genes.
-
Conjugation: This is the closest thing bacteria have to sex. Two bacteria physically connect, and one directly transfers a plasmid (a small, circular piece of DNA) to the other. Plasmids often carry genes for antibiotic resistance, virulence factors, or other advantageous traits.
Beyond the Basics: Emerging HGT Pathways
Scientists are continually uncovering new and unexpected ways that bacteria exchange genes. For example, some bacteria can directly absorb double-stranded DNA from their surroundings, a process that challenges previous assumptions about the requirements for DNA uptake. Other mechanisms involve small vesicles or nanotubes that allow for direct transfer of genetic material between cells. These discoveries show us how complex and adaptable bacterial gene transfer can be.
Mobile Genetic Elements: The Ultimate Facilitators
Mobile genetic elements (MGEs) are specialized DNA sequences that can move from one location to another within a bacterium’s genome, or even between different bacteria. These include transposons (“jumping genes”), insertion sequences, and integrons. MGEs often carry valuable genes, including those for antibiotic resistance, and their ability to move around significantly accelerates the spread of genetic material. They are key players in bacterial evolution and adaptation.
High Stakes: Antibiotic Resistance and Other Impacts
HGT is a major driver of antibiotic resistance, which is a serious global threat. The ability of bacteria to quickly share resistance genes means that even if resistance develops in one isolated location, it can rapidly spread to other bacteria, even across species. This creates an evolutionary arms race between humans and bacteria, where we develop new antibiotics, and bacteria evolve resistance to them. Understanding and controlling HGT is essential to slowing down this process. HGT also influences the pathogenicity (disease-causing ability) of bacteria, the ability to metabolize different compounds, and the overall structure and function of microbial communities.
Combating the Spread of Resistance: A Multi-pronged Approach
Because HGT plays a critical role in the spread of antibiotic resistance, understanding its details—how genes are exchanged, how often, and under what conditions—is essential for developing effective strategies to combat this threat. This requires a collaborative effort from multiple stakeholders, including public health officials, researchers, pharmaceutical companies, and the agricultural industry. Furthermore, continued investigation into non-canonical pathways and novel control strategies are needed.
Here’s how different players can contribute:
| Stakeholder | Short-Term Actions | Long-Term Actions |
|---|---|---|
| Public Health Officials | Improve antibiotic stewardship programs; enhance surveillance of antibiotic resistance patterns. | Develop effective public health policies regulating antibiotic use; invest in research into novel control strategies and alternative therapies. |
| Pharmaceutical Companies | Develop new antibiotics and phage therapies; screen for compounds that inhibit HGT proteins. | Invest in research focused on novel drug development strategies; explore alternative therapies to antibiotics. |
| Research Scientists | Investigate the mechanisms of HGT, including dsDNA uptake, vesicle transfer, and the role of MGEs. | Develop models to better predict the spread of antibiotic resistance genes; explore non-canonical pathways for HGT; investigate the ecology of HGT. |
| Agricultural Industry | Minimize antibiotic use in animal agriculture; improve biosecurity measures. | Implement sustainable livestock disease management strategies; develop novel prophylactic approaches, such as vaccines and probiotics. |
| Environmental Scientists | Monitor antibiotic resistance genes in soil and water; develop methods to remove ARGs from the environment. | Investigate the impact of pollution on HGT; develop strategies to reduce the spread of antibiotic resistance genes in the environment. |
The Future of Bacterial Genetics: An Ongoing Exploration
The study of HGT is a rapidly evolving field, and new discoveries are constantly reshaping our understanding of bacterial evolution. As our knowledge improves, so will our ability to develop strategies to control antibiotic resistance and harness the power of HGT for beneficial purposes in biotechnology and environmental remediation. Could this knowledge revolutionize personalized medicine strategies by allowing us to manipulate bacterial communities in the gut to improve health?
How to Inhibit Bacterial Horizontal Gene Transfer of Antibiotic Resistance Genes: A Complex Challenge
Key Takeaways:
- Bacteria share antibiotic resistance genes (ARGs) through horizontal gene transfer (HGT), particularly within biofilms.
- Biofilms act as hotspots, accelerating the spread of resistance compared to free-floating bacteria.
- Three major HGT mechanisms—conjugation, transformation, and transduction—operate within biofilms.
- Less-understood mechanisms, like outer membrane vesicle (OMV) transfer and nanotubes, also contribute.
- Environmental factors significantly influence HGT rates within biofilms.
- In-depth research is crucial to understand and control HGT.
- Developing strategies to inhibit bacterial horizontal gene transfer of antibiotic resistance genes requires multi-pronged approaches.
The Biofilm Fortress: A Perfect HGT Breeding Ground
Biofilms are complex communities of bacteria encased in a self-produced matrix of extracellular polymeric substances (EPS). Within biofilms, bacteria are in close proximity, nutrients are concentrated, and genetic material can be readily exchanged. The biofilm matrix also protects bacteria from antibiotics and disinfectants, further promoting the spread of resistance.
Three Main Ways Bacteria Share Resistance within Biofilms:
- Conjugation: Direct transfer of plasmids containing resistance genes between bacteria in close contact. The biofilm matrix facilitates this contact.
- Transformation: Bacteria take up free-floating DNA fragments containing resistance genes from the surrounding environment. The biofilm matrix can concentrate DNA, increasing the likelihood of transformation.
- Transduction: Bacteriophages transfer resistance genes between bacteria. Biofilms can promote phage replication and the spread of transducing particles.
Beyond the Big Three: Other HGT Mechanisms in Biofilms
Outer membrane vesicles (OMVs), nanoscale sacs secreted by bacteria, can transport DNA, proteins, and other molecules between cells. Nanotubes, tiny channels that connect bacterial cells, can also facilitate the direct transfer of genetic material. These mechanisms add another layer of complexity to HGT in biofilms.
How to Inhibit Bacterial Horizontal Gene Transfer of Antibiotic Resistance Genes: Challenges and Strategies
Stopping the spread of resistance requires a multifaceted approach that targets HGT mechanisms directly, disrupts biofilm formation, promotes responsible antibiotic use, and develops new antibiotics and alternative therapies.
Specific strategies include:
- Targeting HGT mechanisms directly:
- Developing inhibitors that block conjugation, transformation, and transduction.
- Interfering with the production or function of OMVs and nanotubes.
- Disrupting biofilm formation:
- Using new materials that inhibit bacterial adhesion and biofilm formation on medical devices.
- Developing enzymes that degrade the biofilm matrix.
- Antibiotic stewardship:
- Implementing programs to promote the appropriate use of antibiotics in human and animal medicine.
- Reducing the selective pressure for resistance.
- Improved sanitation and wastewater treatment:
- Removing antibiotic resistance genes and bacteria from wastewater.
- Preventing the spread of resistance in the environment.
- Developing new antibiotics and alternatives:
- Discovering new antibiotics with novel mechanisms of action.
- Exploring alternative therapies, such as phage therapy, antimicrobial peptides, and probiotics.
The Path Forward: Research and Collaboration
Despite advances, significant unknowns surrounding HGT remain. Further research is needed to fully understand the mechanisms of HGT, identify new targets for intervention, and develop effective strategies to combat AMR. This requires collaboration between researchers, clinicians, public health officials, and industry.
Horizontal Gene Transfer Mechanisms in Diverse Bacterial Species
Key Takeaways:
- Horizontal gene transfer (HGT) is a primary driver of bacterial evolution and adaptation.
- Three primary HGT mechanisms exist: conjugation, transformation, and transduction.
- Precisely measuring HGT rates in natural settings remains challenging.
- The fitness impact of transferred genes varies greatly depending on the environment.
- Understanding HGT is crucial for managing antibiotic resistance and understanding bacterial evolution.
Conjugation: A Close Encounter
Conjugation occurs when two bacterial cells come into direct contact, and one cell transfers genetic material (usually a plasmid) to the other. This process is often mediated by a sex pilus, a protein appendage that extends from the donor cell and attaches to the recipient cell. Conjugation is particularly important for the spread of antibiotic resistance genes, as plasmids often carry multiple resistance genes.
Transformation: DNA Uptake from the Environment
Transformation occurs when bacteria take up free DNA from their environment. This DNA may be released from dead bacteria or may be present in the environment as a result of other processes. For transformation to occur, the recipient bacterium must be “competent,” meaning that it has the ability to bind and import DNA.
Transduction: Viral Hitchhikers
Transduction involves bacterial viruses (bacteriophages) as gene couriers. When a phage infects a bacterium, it may accidentally package up a piece of the bacterium’s DNA instead of its own. When this phage infects another bacterium, it injects this DNA, potentially introducing new genes.
The Impact of HGT: Antibiotic Resistance and Beyond
The rapid spread of antibiotic resistance genes via HGT is a major challenge for global public health. However, HGT also contributes to bacterial adaptation in various environments, allowing bacteria to acquire new metabolic capabilities, virulence factors, and other traits that enhance their survival and competitiveness.
Unraveling the Mysteries: Ongoing Research and Future Directions
While we understand the basic mechanisms of HGT, much remains unknown about the frequency, efficiency, and ecological consequences of HGT in natural environments. Researchers are working on developing new tools and techniques to measure HGT rates, identify the factors that influence HGT, and predict the long-term impacts of HGT on bacterial evolution and adaptation.
Environmental Factors Influencing Horizontal Gene Transfer Rates
Key Takeaways:
- Soil bacteria readily share antibiotic resistance genes (ARGs).
- Horizontal gene transfer (HGT), particularly through transformation, is a major mechanism for the spread of ARGs in soil.
- Environmental Factors Influencing Horizontal Gene Transfer Rates, such as soil composition, land use practices, and the presence of pollutants, significantly impact ARG prevalence.
- The proximity of Listeria strains to L. monocytogenes correlates with higher ARG counts, suggesting that HGT is more likely to occur between closely related strains.
- Understanding the interplay between HGT and environmental pressures is crucial for managing antibiotic resistance.
The Soil’s Influence: A Microbial Hotspot
Soil is a complex and dynamic environment that harbors a vast diversity of microorganisms, including bacteria, fungi, and viruses. Soil bacteria play a critical role in nutrient cycling, decomposition, and other essential ecosystem processes. However, soil is also a reservoir of antibiotic resistance genes (ARGs), which can be transferred to other bacteria, including human pathogens.
The Power of the Environment
Specific soil properties, such as pH, moisture content, and the presence of organic matter, can influence the survival and activity of bacteria, as well as the rate of HGT. Land use practices, such as agriculture and urbanization, can also impact ARG prevalence by introducing antibiotics, heavy metals, and other pollutants into the soil.
Transformation Takes the Lead in Soil
Transformation is thought to be a particularly important mechanism for HGT in soil, as bacteria are constantly exposed to free DNA released from dead cells. However, conjugation and transduction can also occur in soil, particularly in biofilms and other microenvironments where bacteria are in close proximity.
Implications and Future Directions
The findings have crucial implications for public health and environmental management. The fact that ARGs are easily swapped amongst Listeria and other soil bacteria underscores the importance of careful monitoring and proactive strategies to minimize the spread of antibiotic resistance. This includes reducing antibiotic use in agriculture, improving wastewater treatment, and promoting sustainable land management practices.