Unlock stoichiometry success! Master balancing chemical equations with our comprehensive collection of printable worksheets, video tutorials, and practice problems, tailored for KS3, GCSE, and beyond. From reactants to products, learn the fundamental principles and apply them with confidence using our diverse range of resources, including interactive exercises and answer keys. Ready to boost your chemistry grades and go beyond the basics? Let’s dive in!
What is a Chemical Equation?
Think of a chemical equation as a shorthand recipe for a chemical reaction. Just like a recipe lists ingredients and instructions, a chemical equation uses symbols and formulas to represent the substances involved and how they transform. Let’s break it down:
- Reactants: These are the starting materials, like the ingredients in your recipe. They’re written on the left side of the equation.
- Products: These are the substances formed during the reaction, like the delicious cake you bake. They’re written on the right side of the equation.
- Arrow (→): This symbol separates the reactants and products, indicating the direction of the chemical change. It’s like the oven transforming your batter into a cake!
For example, H₂ + O₂ → H₂O represents the reaction of hydrogen (H₂) and oxygen (O₂) to form water (H₂O). Simple, right?
Why Balance Equations? The Law of Conservation of Mass
Now, imagine baking a cake but forgetting half the flour. Disaster, right? In chemistry, we have a similar principle called the Law of Conservation of Mass. This fundamental law states that matter cannot be created or destroyed during a chemical reaction; it can only be rearranged. It’s like LEGOs – you can build different structures, but the total number of LEGO bricks stays the same.
Balancing a chemical equation ensures we account for every single atom involved. The number of atoms of each element must be the same on both sides of the equation. This isn’t just about neatness; it’s about accurately representing what happens at the atomic level. A balanced equation reflects the Law of Conservation of Mass in action.
Balancing Equations: Your Step-by-Step Guide
One of the most effective methods for balancing equations is the inspection method. It’s like solving a puzzle: you adjust the “amounts” of each molecule until everything fits perfectly. These “amounts” are indicated by coefficients, the numbers placed in front of the chemical formulas. Here’s the step-by-step process:
- Double-Check Formulas: Before balancing, ensure all chemical formulas are correct. A small error here can lead to big problems later.
- Start Simple: Begin with an element that appears in the fewest number of molecules on either side of the equation.
- Adjust Coefficients: Use coefficients to balance the chosen element. Never change subscripts! Changing subscripts alters the chemical identity of the substance, which isn’t allowed!
- Iterate: Move to the next element and repeat step 3. You might need to go back and forth a few times until everything is balanced.
- Final Check: Double-check your work. Make sure the number of atoms of each element is identical on both sides.
Let’s balance the equation for the combustion of methane (CH₄): CH₄ + O₂ → CO₂ + H₂O.
- Formulas are correct.
- Carbon is already balanced (1 on each side).
- Balance hydrogen: CH₄ + O₂ → CO₂ + 2H₂O (now 4 hydrogens on each side).
- Balance oxygen: CH₄ + 2O₂ → CO₂ + 2H₂O (now 4 oxygens on each side).
- Final check confirms the equation is balanced!
Types of Chemical Reactions: A Balancing Overview
Balancing is essential for all types of chemical reactions. Here are a few common ones:
- Synthesis (A + B → AB): Two or more substances combine to form a more complex product.
- Decomposition (AB → A + B): A complex substance breaks down into simpler ones.
- Single Displacement (A + BC → AC + B): One element replaces another in a compound.
- Double Displacement (AB + CD → AD + CB): Two compounds exchange ions.
- Combustion (Fuel + O₂ → CO₂ + H₂O): A substance reacts with oxygen, often producing heat and light.
Each reaction type presents unique balancing challenges, but the core principles remain the same.
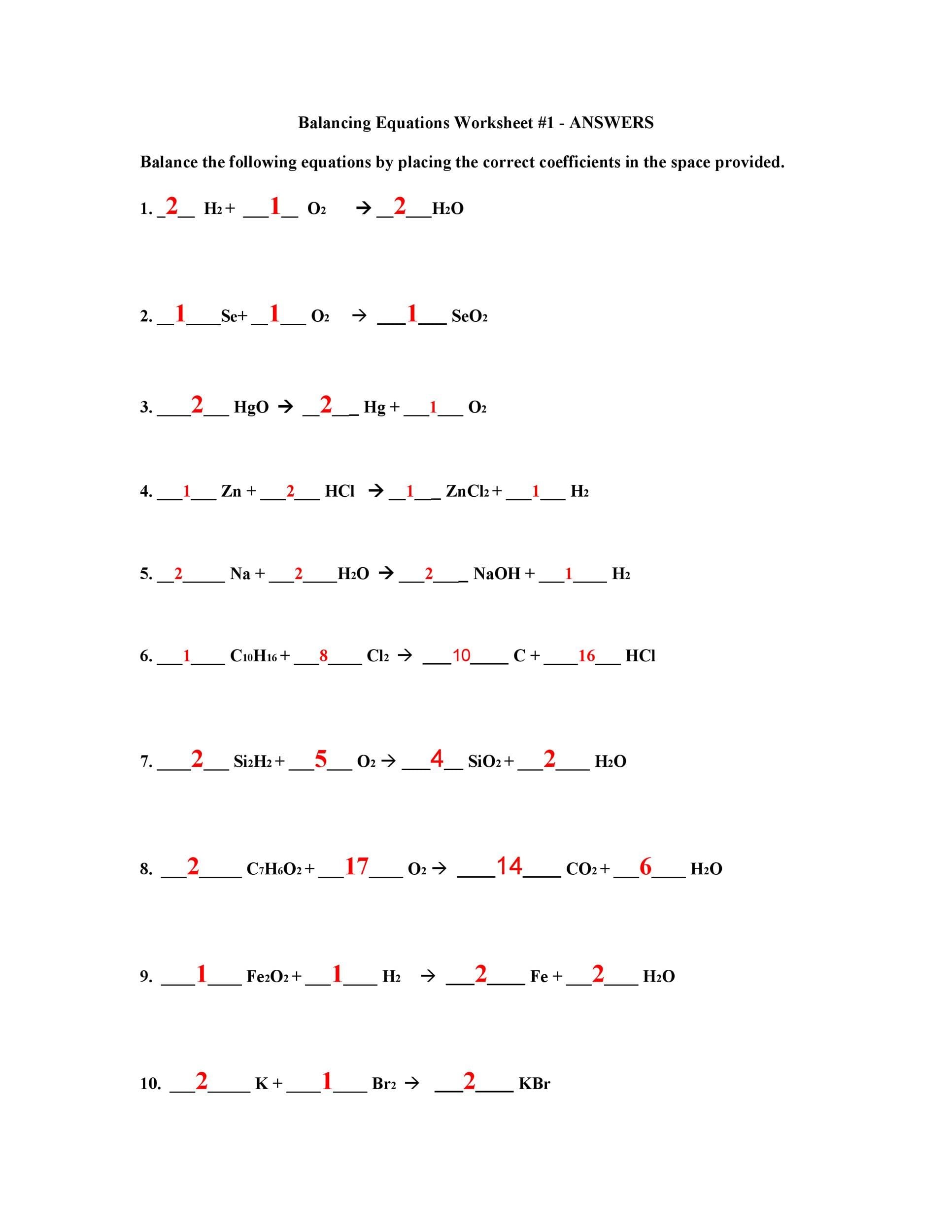
Interactive Practice and Worksheets
Practice makes perfect! Our science balancing equations worksheets offer a range of problems to hone your skills, with answer keys for self-assessment. We also have interactive exercises that provide instant feedback, helping you identify and correct errors as you learn. Downloadable versions categorized by difficulty level (KS3, GCSE, and beyond) are also available.
Video Tutorials: Visualizing Balancing
Visual learners, rejoice! We have short video tutorials demonstrating balancing techniques, similar to Khan Academy and Crash Course, making complex concepts easier to grasp. These videos break down the process step-by-step, offering clear explanations and helpful examples.
Common Mistakes to Avoid
Even seasoned chemists make mistakes! Here are a few common pitfalls to watch out for:
- Changing Subscripts: Never change subscripts! This changes the identity of the substance. Only adjust coefficients.
- Ignoring Coefficients of 1: A blank space in front of a molecule implies a coefficient of 1. Don’t forget to account for these!
Real-World Applications: Beyond the Classroom
Balancing equations isn’t just an academic exercise; it’s a vital tool in countless fields. From calculating the correct proportions of reactants in industrial processes to understanding the reactions that sustain life, balancing equations has real-world significance. It also underlies stoichiometry, which allows chemists to predict the quantities of reactants and products in a reaction.
Advanced Balancing: Redox Reactions and More
Ready for a challenge? Explore advanced balancing techniques for more complex equations, such as redox reactions, which involve the transfer of electrons. These reactions can be tricky to balance, but with practice, you can master even the most challenging problems.
Additional Resources: Keep Exploring!
Want to learn more? Check out these additional resources:
- Online Balancing Equation Checkers: Verify your work with online tools.
- Interactive Tutorials: Explore interactive tutorials for further practice.
- Further Reading: Delve deeper into chemical reactions and balancing concepts with recommended reading materials.
Quickly assess your students’ phonics skills with our comprehensive quick phonics screener. Explore the innovative world of education with our cutting-edge teachy ai platform.
By following these guidelines and utilizing these resources, you’ll master balancing chemical equations in no time. Remember, practice is key, and the more you work with equations, the more intuitive the process becomes. While the principles we’ve discussed are generally accepted, ongoing research in chemistry suggests that our understanding of chemical reactions may continue to evolve. So, embrace the journey of learning, and keep exploring the fascinating world of chemistry!