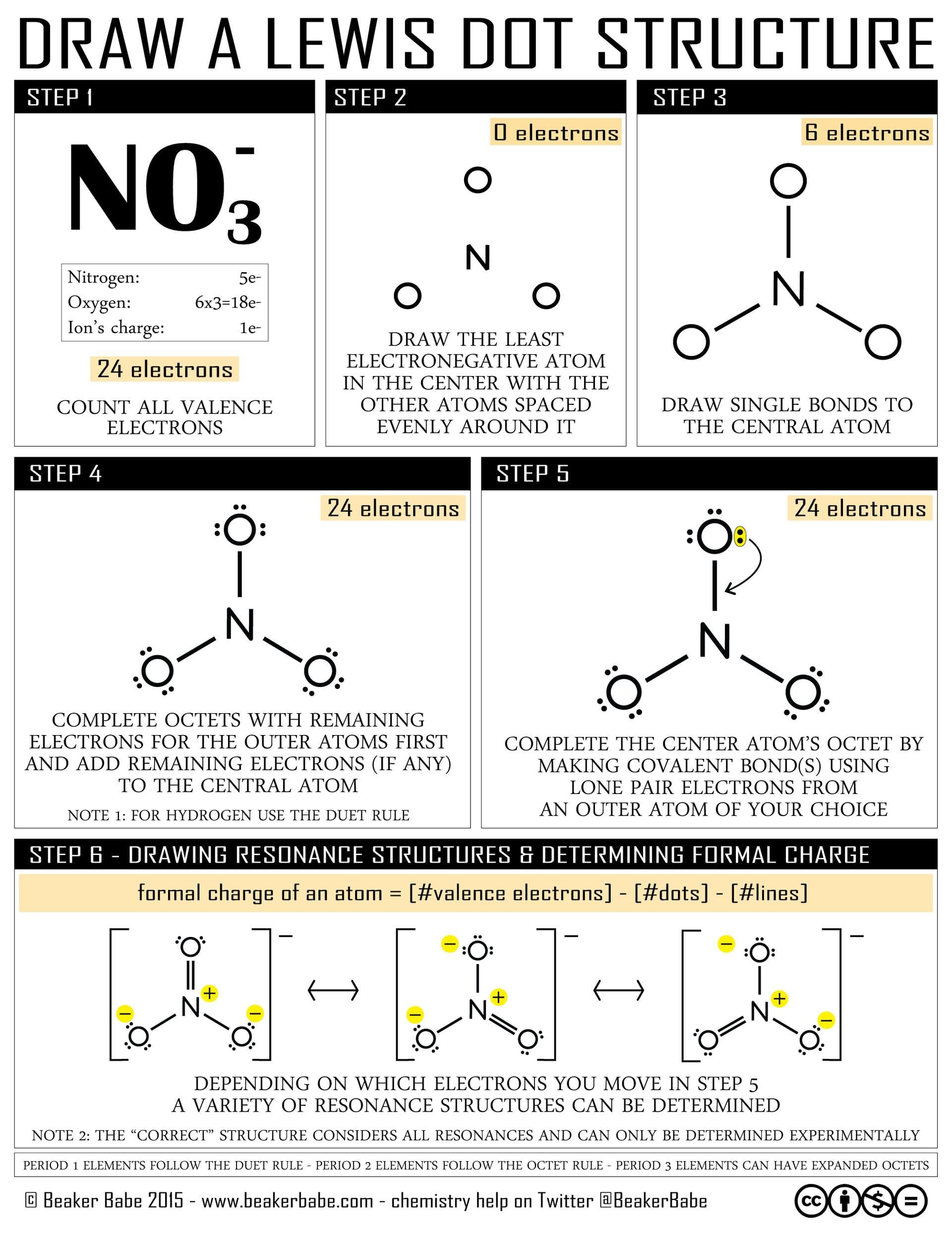
This guide provides a comprehensive walkthrough of constructing the Lewis structure for the sulfur trifluoride anion (SF3-), explaining its shape, hybridization, and properties.
Understanding SF3- Basics
SF3-, also known as the sulfur trifluoride anion, consists of one sulfur atom and three fluorine atoms, carrying an overall negative charge. The Lewis structure helps visualize how valence electrons participate in bonding and determine the molecule’s shape. To gain a deeper understanding of another molecule’s structure, you can explore the Lewis dot for HCN.
Constructing the SF3- Lewis Structure
Counting Valence Electrons
- Sulfur (S): Group 16, contributes 6 valence electrons.
- Fluorine (F): Group 17, each of the three fluorine atoms contributes 7 valence electrons (21 total).
- Negative Charge: Adds 1 extra electron.
Total valence electrons: 6 + 21 + 1 = 28
Building the Framework
- Central Atom: Sulfur, being less electronegative than fluorine, occupies the central position.
- Single Bonds: Connect each fluorine atom to the sulfur with a single bond, using 2 electrons per bond (6 total).
Completing Octets
- Fluorine Octets: Each fluorine atom needs 6 more electrons to complete its octet. Add 3 lone pairs (6 electrons) to each fluorine (18 total).
Assigning Remaining Electrons
- Sulfur’s Lone Pair: Place the remaining 2 electrons as a lone pair on the sulfur atom. Sulfur now has 10 electrons around it (an expanded octet), which is possible for elements in the third period and beyond.
Formal Charge
- Sulfur: 6 (valence) – 2 (lone pair) – 3 (bonds) = +1. Since the ion has a -1 charge, an additional electron is added to sulfur, resulting in a -1 formal charge on sulfur, consistent with the overall charge.
Key Points of Sulfur Trifluoride Anion (SF3-) Lewis Structure:
- Valence Electrons: 28 (6 from S, 21 from 3 F, and 1 negative charge)
- Central Atom: Sulfur (S)
- Bonding: Three single bonds between S and F
- Octet Rule Fulfillment: Each F atom has eight valence electrons (octet)
- Lone Pair: One lone pair of electrons on S
- Formal Charge: Sulfur has a formal charge of -1, matching the overall ion charge
- Geometry: Trigonal pyramidal, with lone pair repelling bonding pairs
- Reactivity: Lone pair and negative formal charge suggest potential electron donation
- Variations: SF3+ (cation) and neutral SF3 exist with different properties
SF3- Molecular Geometry: The Trigonal Pyramid
Visualizing the 3D Structure
The SF3- ion exists as a 3D structure. The sulfur atom is at the apex of a trigonal pyramid, with the three fluorine atoms forming the base. The lone pair on sulfur occupies space and repels the bonding pairs, leading to a bond angle slightly less than the ideal 109.5° of a tetrahedron (probably around 105°).
SF3- Hybridization: sp3
Orbital Mixing
The sulfur atom in SF3- undergoes sp3 hybridization. One s orbital and three p orbitals combine to form four equivalent sp3 hybrid orbitals. Three of these orbitals form sigma bonds with the fluorine atoms, while the fourth orbital accommodates the lone pair of electrons.
Impact on Geometry
The sp3 hybridization explains the approximate tetrahedral arrangement of electron pairs around sulfur. While the electron pair geometry is roughly tetrahedral, the molecular geometry (considering only the atoms) is trigonal pyramidal.
Comparing SF3- to Other Sulfur Fluorides
SF3- stands in contrast to both neutral SF3 and SF3+. Neutral SF3, if it exists, likely has a different geometry and reactivity due to the absence of a formal charge. SF3+ would be even more distinct due to the positive charge on sulfur, significantly altering its electron configuration and bonding behavior.
Further Exploration and Ongoing Research
Understanding the Lewis structure, geometry, and hybridization of SF3- forms the basis for predicting its reactivity and interactions with other molecules. Research continues to refine our understanding of the precise nature of bonding in SF3-, exploring nuances such as the potential involvement of d-orbitals, a topic of ongoing debate in the scientific community. This research highlights the dynamic nature of scientific knowledge and the importance of continuously questioning and refining our models.














1 thought on “Drawing the SF3Lewis Structure: A Step-by-Step Tutorial & Common Mistakes”
Comments are closed.