Ever wondered why salt dissolves in water but oil doesn’t? The answer lies in the fascinating world of ionic bonding. This comprehensive guide explores ionic bonding, from fundamental concepts to advanced principles, using worksheets and interactive resources to enhance your learning. Whether you’re a GCSE student, an aspiring chemist, or simply curious about the world around you, this guide will equip you with the knowledge and tools to master ionic bonding.
Decoding Ionic Bonds
What is Ionic Bonding?
Ionic bonding is the electrostatic attraction between oppositely charged ions. These ions, called cations (positive) and anions (negative), are formed when atoms transfer electrons. Think of it as a tiny game of give-and-take at the atomic level. Typically, a metal atom generously donates its outermost electron(s) to a non-metal atom. This exchange creates the opposing charges that draw the ions together, forming a strong ionic bond. This bond is the reason behind the unique properties of ionic compounds, such as the way table salt (sodium chloride) dissolves in water or forms distinct crystals.
Why Use Worksheets?
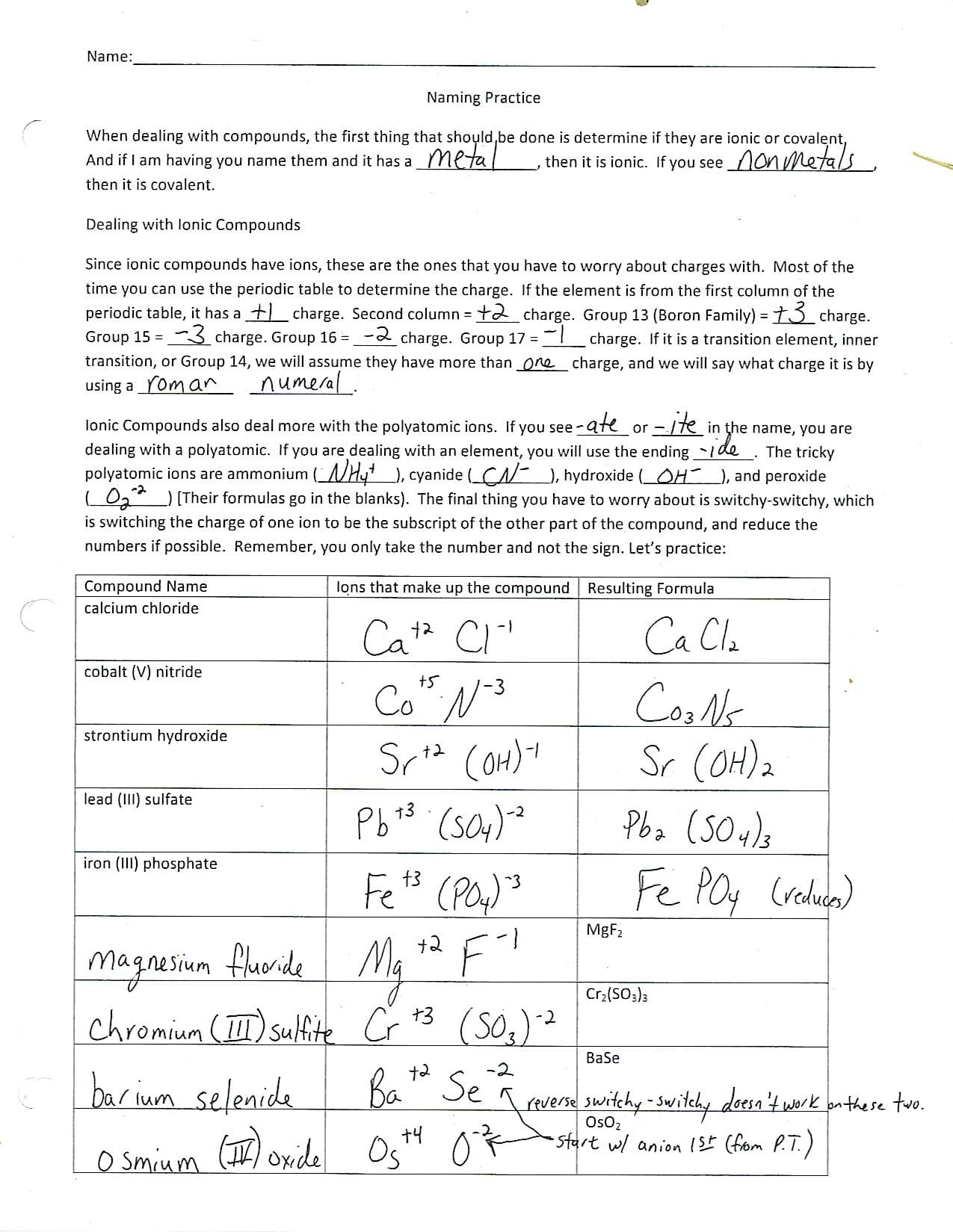
Ionic bonding worksheets are valuable tools for visualizing and understanding this fundamental chemical concept. They provide a structured approach to learning, regardless of your current level. They offer:
- Visual Practice: Dot-and-cross diagrams on worksheets help you see how electrons are transferred and how ionic compounds form.
- Formula Mastery: Worksheets can guide you in writing chemical formulas for ionic compounds, which is essential for communicating chemical information.
- Conceptual Depth: Some worksheets even explore concepts like Johnstone’s Triangle, bridging the gap between the microscopic world of atoms and the macroscopic properties we observe.
- Self-Paced Learning: Worksheets allow you to learn at your own speed, making mistakes, and reviewing concepts until you’ve mastered them.
Exploring Ionic Bonding Worksheets
Ionic bonding worksheets cater to a wide range of topics and skill levels:
- Beginner: Focus on basic electron transfer, introducing dot-and-cross diagrams and simple ionic compound formation.
- Intermediate: Cover writing formulas for ionic compounds, predicting ion charges, and balancing ionic equations.
- Advanced: Delve into Johnstone’s Triangle, lattice energy, the Born-Haber cycle, and comparisons with covalent bonding.
Ready to tackle more challenging algebraic expressions? Dive into our comprehensive multiplying polynomials worksheet for targeted practice.
Worksheet Types and Resources
- Dot-and-Cross Diagrams: These diagrams visually represent the arrangement of electrons in atoms and ions, making electron transfer easier to understand.
- Formula Writing: Practice writing chemical formulas for ionic compounds, using the charges of ions to determine the correct ratios.
- Ionic Equations: Learn to write and balance ionic equations, which represent chemical reactions involving ions.
- Johnstone’s Triangle: Explore this powerful tool to connect the macroscopic properties of ionic compounds (like melting point and solubility) to their microscopic structure.
Finding Worksheets: Reputable websites like Tes and the Royal Society of Chemistry (RSC) Education offer a wealth of free printable worksheets, often with accompanying answer keys. Twinkl is another good resource, offering a range of differentiated worksheets to accommodate different levels of learning.
Using Worksheets Effectively
For Students
- Start Simple: Begin with easier exercises to build confidence before tackling more challenging concepts.
- Visualize: Use dot-and-cross diagrams to picture the electron transfer process.
- Practice: Regular practice with worksheets reinforces learning and helps you identify areas where you need more help.
- Check Answers: Use answer keys to identify mistakes and understand the correct reasoning.
- Seek Help: Don’t hesitate to ask your teacher or peers for help when needed.
For Educators
- Lesson Integration: Incorporate worksheets into lesson plans for in-class practice or homework assignments.
- Differentiation: Choose worksheets that cater to different learning styles and levels of understanding.
- Assessment: Use worksheets for both formative and summative assessments.
- Discussion Starters: Use worksheets as a springboard for class discussions about ionic bonding concepts.
Simplify your fractions with ease using our interactive reducing fractions worksheet and strengthen your core math skills.
Johnstone’s Triangle Explained
Johnstone’s Triangle is a powerful tool for connecting different levels of chemical representation:
- Macroscopic: The observable properties of a substance (e.g., the fact that salt is a crystalline solid).
- Sub-microscopic: The arrangement and behavior of atoms and ions (e.g., the arrangement of sodium and chloride ions in a crystal lattice).
- Symbolic: The chemical formulas and equations used to represent substances and reactions (e.g., the formula NaCl for sodium chloride).
Johnstone’s Triangle helps you see how these levels are interconnected. For example, the strong electrostatic attraction between ions (sub-microscopic) explains why ionic compounds have high melting points (macroscopic), which is represented by the chemical formula (symbolic).
Beyond GCSE: Advanced Concepts
- Lattice Energy: This measures the strength of the ionic bond, reflecting the energy required to separate ions in a crystal lattice.
- Born-Haber Cycle: This thermodynamic cycle analyzes the energy changes involved in forming an ionic compound from its elements.
Ionic vs. Covalent Bonding
Ionic and covalent bonding are the two primary types of chemical bonds. The key difference lies in how electrons are involved:
| Feature | Ionic Bond | Covalent Bond |
|---|---|---|
| Electron Behavior | Complete transfer | Sharing of electrons |
| Electronegativity | Large difference | Small difference |
| Typical Properties | High melting/boiling points, brittle, often conductive in solution | Lower melting/boiling points, may be gases, liquids, or solids, often not conductive in solution |
Interactive Learning
Online simulations and quizzes can make learning about ionic bonding more engaging and interactive. Explore resources like PhET Interactive Simulations for dynamic visualizations of chemical processes.
Conclusion
Ionic bonding is a fundamental concept in chemistry. Worksheets, combined with interactive resources and a clear understanding of concepts like Johnstone’s Triangle, provide powerful tools for mastering this essential topic. By exploring the various types of worksheets and utilizing them effectively, you can build a strong foundation for future studies in chemistry and gain a deeper appreciation for the world around you.
- Unraveling Einstein’s Legacy: Who Inherited His Genius? - July 14, 2025
- Unlock Einstein’s Family Tree: Bernhard Caesar & Untold Stories - July 14, 2025
- Unveiling Bernhard Caesar Einstein: His Life & Albert Einstein’s Legacy - July 14, 2025