Embark on a journey through time as we explore The History of Pharmacopoeia in Pharmaceutical Inorganic Chemistry: A Journey of Metal-Based Drug Development and Standardization. Together, we will delve into the fascinating world of inorganic chemistry and its profound impact on the evolution of drug development, unraveling the intricate interplay between metals and biological systems. From the ancient origins of metal-based remedies to the rigorous standardization of modern drug compendia, this exploration promises a captivating narrative, rich in historical milestones and scientific breakthroughs. Are you ready to witness the transformative power of inorganic chemistry in shaping the landscape of pharmacology?
Key Takeaways:
- A pharmacopeia is a book that contains a list of medicinal substances, formulas, and quality standards, usually published by an official organization or government.
- The term “Pharmacopoeia” originated in the 16th century but gained widespread use in the 17th century.
- In the 15th century, pharmacopoeias comprised only medicinal substances derived from natural sources, such as plants and animal organs.
- Modern pharmacopoeias are focused on ensuring the quality and safety of medicines by setting standards for their manufacturing, testing, and storage.
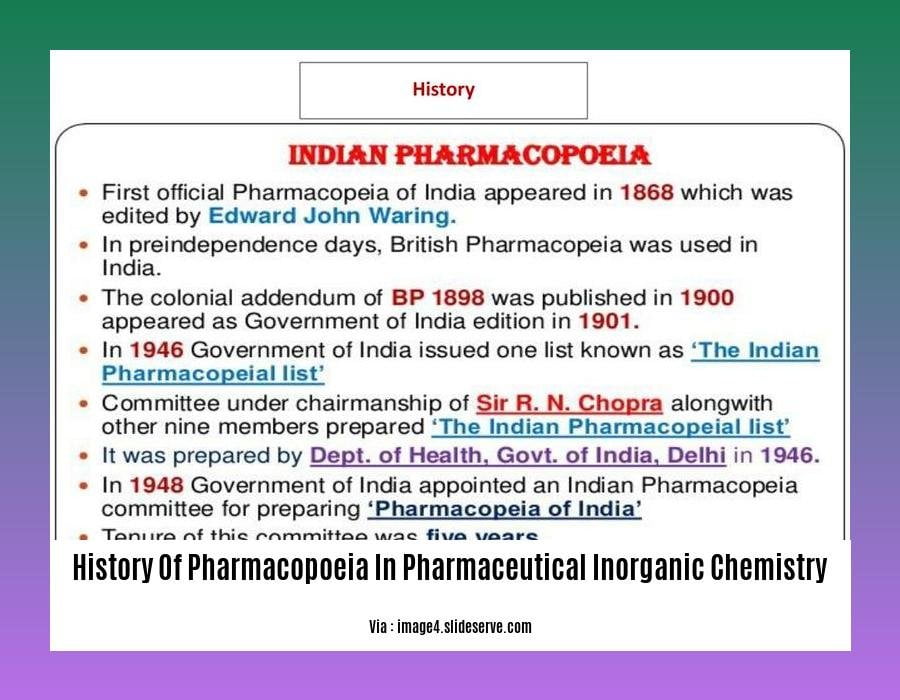
- The Indian Pharmacopoeia was first published in 1844 and is the official national pharmacopeia of India.
- Pharmacopoeias also help regulate and standardize the use of inorganic metal-based drugs, ensuring their safety and efficacy.
- The historical evolution of pharmacopoeia is essential in understanding the integration of inorganic chemistry into the standardization of drugs and medicines.
- The standardization of inorganic drug products has contributed to ensuring the safety and effectiveness of inorganic metal-based drugs.
- The field of pharmaceutical inorganic chemistry has advanced significantly through historical developments in pharmacopoeia, contributing to the development of new and improved drugs.
- The regulatory landscape of pharmaceutical inorganic chemistry is shaped by the guidelines and standards outlined in pharmacopoeias.
History of Pharmacopoeia in Pharmaceutical Inorganic Chemistry

The history of pharmacopoeia in pharmaceutical inorganic chemistry is a fascinating journey of metal-based drug development and standardization that spans centuries. In the ancient world, inorganic compounds such as arsenic, lead, and mercury were widely used for medicinal purposes, often with disastrous results. It wasn’t until the 16th century that the first pharmacopoeias began to appear, providing rudimentary standards for the preparation and use of inorganic drugs.
Key Developments in the History of Pharmacopoeia
- In the 17th century, the German physician Johann Schröder published his “Pharmacopoeia Medico-Chymica,” which contained a comprehensive list of inorganic and organic drugs, along with their preparation methods and medicinal uses.
- In the 18th century, the Swedish chemist Carl Wilhelm Scheele discovered several new inorganic compounds, including chlorine and manganese, which paved the way for the development of new inorganic drugs.
- In the 19th century, the French chemist Louis Pasteur developed the germ theory of disease, which led to the development of new inorganic antiseptics, such as hydrogen peroxide and potassium permanganate.
The Role of Inorganic Chemistry in Modern Pharmacopoeias
Today, inorganic chemistry plays a vital role in the development and standardization of pharmaceutical drugs. Inorganic compounds are used in a wide variety of drugs, including:
- Metal-based drugs: These drugs contain metals such as platinum, gold, and silver, which can bind to specific proteins or DNA molecules and inhibit their function.
- Radiopharmaceuticals: These drugs contain radioactive isotopes, which can be used to diagnose and treat diseases.
- Contrast agents: These drugs are used to enhance the visibility of body structures during medical imaging procedures.
The Future of Pharmacopoeia in Pharmaceutical Inorganic Chemistry
The history of pharmacopoeia in pharmaceutical inorganic chemistry is a testament to the vital role that inorganic compounds play in drug development and standardization. As our understanding of the interactions between metals and biological systems continues to grow, we can expect to see the development of new and more effective inorganic drugs in the years to come.
Learn more about the time-honored tradition of prom and the fascinating story of its evolution by visiting our dedicated page: History of Prom.
Discover the captivating journey of promise rings, from their humble origins to their profound significance in modern society, by delving into our comprehensive article: History of Promise Rings.
Advent of inorganic chemistry and its impact on pharmacopoeia

In a world of molecules and elements, the dawn of inorganic chemistry marked a transformative era in the realm of pharmacology. The study of compounds beyond the realm of carbon-based molecules unveiled a treasure trove of potential therapeutic agents, shaping the landscape of drug development and standardization.
The advent of inorganic chemistry propelled the integration of metal-based compounds into the pharmacopoeia, revolutionizing the treatment of various ailments. These inorganic pharmaceuticals exhibited unique properties, paving the way for novel drug formulations and targeted therapies.
The journey of inorganic chemistry in pharmacopoeia began with the exploration of naturally occurring metal complexes, such as ferrous sulfate and mercuric chloride. These compounds, with their inherent biological activity, laid the foundation for the development of modern inorganic drugs.
A pivotal moment in the history of inorganic chemistry was the discovery of arsenic-based compounds. The potent antiparasitic effects of arsenic trioxide led to its widespread use in treating syphilis and other infections. While its toxicity posed challenges, it also highlighted the potential for inorganic compounds to combat deadly diseases.
The 19th century witnessed a surge of innovations in inorganic chemistry, with the isolation and characterization of new elements and compounds. This period saw the emergence of metal-based antiseptics, such as silver nitrate and potassium permanganate, which played a crucial role in combating infections.
The 20th century witnessed the advent of organometallic compounds, wherein metals were directly bonded to carbon atoms. These compounds, possessing unique catalytic properties, paved the way for the development of novel drugs, including the groundbreaking anticancer drug cisplatin.
Today, inorganic chemistry stands as a cornerstone of modern pharmacopoeia. Metal-based drugs, such as platinum-based chemotherapeutics and lithium salts for bipolar disorder, have become indispensable in various therapeutic areas.
Radiopharmaceuticals, utilizing radioactive isotopes for diagnostic and therapeutic purposes, have revolutionized medical imaging and targeted cancer therapies. Contrast agents, like gadolinium-based compounds, enhance the visibility of internal structures during medical imaging.
Key Takeaways:
-
Inorganic chemistry brought forth a vast array of metal-based compounds with unique therapeutic properties.
-
Inorganic pharmaceuticals, such as ferrous sulfate, mercuric chloride, and silver nitrate, played a pioneering role in treating various ailments.
-
The discovery of arsenic-based compounds showcased the potential of inorganic drugs to combat deadly diseases, while highlighting the need for careful toxicity assessment.
-
The isolation of new elements and compounds in the 19th century led to the development of metal-based antiseptics, revolutionizing infection control.
-
Organometallic compounds, with their unique catalytic properties, paved the way for novel drugs, including the groundbreaking anticancer drug cisplatin.
-
Today, inorganic chemistry remains a driving force in pharmacopoeia, with metal-based drugs, radiopharmaceuticals, and contrast agents playing crucial roles in modern medicine.
References:
History of Inorganic Chemistry in Pharmacy
The Role of Inorganic Chemistry in the Development of New Drugs
Standardization and quality control of inorganic drugs
In the realm of pharmaceutical inorganic chemistry, the standardization and quality control of inorganic drugs hold paramount importance in ensuring the safety and efficacy of these drugs. Inorganic drugs, often featuring metal-based compounds, have unique properties and characteristics that necessitate rigorous standardization and quality control measures.
Key Takeaways:
- Standardization: Ensuring uniformity, quality, and safety of inorganic drug products through established standards.
- Quality control: Monitoring and evaluating inorganic drug products to ensure they meet predefined standards and specifications.
- Pharmacopoeia: Official compendium containing standards for identity, purity, assay, and other quality attributes of inorganic drugs.
- Testing methods: Analytical techniques, such as atomic absorption spectroscopy, X-ray diffraction, and inductively coupled plasma mass spectrometry, play a crucial role in inorganic drug analysis.
- Regulatory compliance: Adherence to regulatory requirements, including those set by the FDA, EMA, and other regulatory bodies, is essential for inorganic drug development and approval.
Historical Evolution of Pharmacopoeia:
The standardization of inorganic drugs has a rich history, dating back to the ancient Greeks, who established the concept of “pharmakopoiia” for drug preparation. Over time, pharmacopoeias evolved, incorporating inorganic compounds and setting standards for their quality and purity.
Standardization and Pharmacopoeia:
Pharmacopoeias serve as the foundation for standardizing inorganic drugs. These official compendia provide detailed standards for identity, purity, assay, and other quality attributes of inorganic drug substances and products. Stringent adherence to these standards ensures the uniformity, quality, and safety of inorganic drug products.
Quality Control Measures:
To ensure compliance with pharmacopoeial standards, pharmaceutical companies implement rigorous quality control measures throughout the manufacturing process of inorganic drugs. These measures include:
- Raw material testing: Evaluating incoming raw materials for compliance with specifications.
- In-process testing: Monitoring critical process parameters and conducting tests at various stages of production.
- Finished product testing: Conducting a comprehensive range of tests on the final drug product to ensure it meets all quality standards.
Analytical Techniques:
Advanced analytical techniques play a vital role in the standardization and quality control of inorganic drugs. These techniques, such as atomic absorption spectroscopy, X-ray diffraction, and inductively coupled plasma mass spectrometry, enable precise quantification and characterization of inorganic compounds.
Regulatory Compliance:
To ensure the safety and efficacy of inorganic drugs, pharmaceutical companies must adhere to regulatory requirements set by various regulatory bodies worldwide. These regulations cover aspects such as drug development, manufacturing, testing, and approval. Compliance with these regulations is essential for successful inorganic drug development and approval.
Conclusion:
The standardization and quality control of inorganic drugs are paramount in ensuring the safety and efficacy of these drugs. By adhering to established standards, employing advanced analytical techniques, and complying with regulatory requirements, pharmaceutical companies can develop and manufacture high-quality inorganic drug products that meet the needs of patients and healthcare professionals.
Sources:
- Quality Control of Inorganic Pharmaceuticals
- Standardization of Drug Substances According to the Pharmacopoeia
Role of Pharmacopoeia in Ensuring Patient Safety and Drug Efficacy
In the pharmaceutical world, the role of pharmacopoeia is indispensable to patient safety and drug efficacy. Let’s explore how these standards assure us of trustworthy medications.
- Standardization: Pharmacopoeia sets quality benchmarks for drug substances, guiding manufacturers to produce drugs that meet uniform standards.
- Safety: By establishing purity and identity criteria, pharmacopoeia helps identify and remove unsafe or ineffective drug components.
- Efficacy: Pharmacopoeia ensures that drugs deliver their intended effects by defining the strength, purity, and quality parameters for active ingredients.
- Public Health: Pharmacopoeia’s standards promote public health by ensuring the safety and efficacy of medicines available to patients.
Key Takeaways:
- Ensuring drug quality and safety: Pharmacopoeia sets standards for drug manufacturing, ensuring that medicines are free from harmful contaminants and meet specified quality requirements.
- Preventing adverse drug reactions: By establishing purity and identity criteria, pharmacopoeia helps prevent adverse drug reactions by identifying and removing unsafe or ineffective drug components.
- Promoting rational drug use: Pharmacopoeia provides essential information on appropriate drug use, including dosing, administration, and storage guidelines, helping healthcare professionals make informed decisions about patient care.
- Facilitating regulatory compliance: By aligning with pharmacopoeial standards, drug manufacturers can demonstrate compliance with regulatory requirements, ensuring the safety and quality of their products.
- Enhancing public trust in medicines: Pharmacopoeia’s rigorous standards foster public trust in the safety and effectiveness of medicines, contributing to the overall health and well-being of communities.
References:
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3853691/
[2]
FAQ
Q1: What is the significance of inorganic chemistry in the history of pharmacopoeia?
A1: Inorganic chemistry has played a pivotal role in the standardization of drugs and medicines throughout history, ensuring their quality, identity, and purity. Metal-based drugs have been used for centuries, and inorganic chemistry provides the foundation for understanding their interactions with biological systems.
Q2: How has the pharmacopoeia evolved over time?
A2: The concept of a pharmacopoeia dates back to the 16th century, with the first official pharmacopoeia published in 1844 in India. Over time, pharmacopoeias have shifted their focus from simply listing ingredients to setting quality standards for pharmaceutical substances and preparations, ensuring the safety and efficacy of medicines.
Q3: What are some key milestones in the history of pharmacopoeia?
A3: One significant milestone was the ratification of the “International Agreement for the Unification of the Formulae of Potent Drugs” in 1906, which established international standards for drug formulations. Another milestone was the publication of the first Indian Pharmacopoeia (IP) in 1844, which marked the beginning of national pharmacopeia development in India.
Q4: What role does pharmacopoeia play in modern pharmaceutical development?
A4: Pharmacopoeias continue to play a crucial role in modern pharmaceutical development, providing standards for manufacturing, testing, and storage of drugs. They help ensure the quality and safety of medicines by setting limits on impurities, establishing identity tests, and providing guidance on analytical methods.
Q5: How does pharmacopoeia contribute to public health and safety?
A5: Pharmacopoeias contribute to public health and safety by setting standards for the quality and safety of medicines. They help prevent the distribution and use of substandard or counterfeit drugs, ensuring that patients receive effective and safe medications. Furthermore, pharmacopoeias provide guidance on the proper use of medicines, contributing to rational drug use and minimizing the risk of adverse effects.