Are you prepared to explore the intriguing realm of condensation polymerization? If you have ever been intrigued by the complex methodology that goes into the formation of polymers, then you have arrived at the appropriate location. This article aims to elucidate intriguing condensation polymerization facts, providing insight into the scientific and engineering processes involved in the development of these multifunctional materials. Drawing upon my extensive experience in chemical engineering and solid foundation in polymer science, I am prepared to offer comprehensive guidance pertaining to the intricacies of optimization techniques, reaction kinetics, and thermodynamics. Therefore, fasten your seatbelts, for we are about to commence an illuminating exploration of condensation polymerization!
Condensation Polymerization Facts
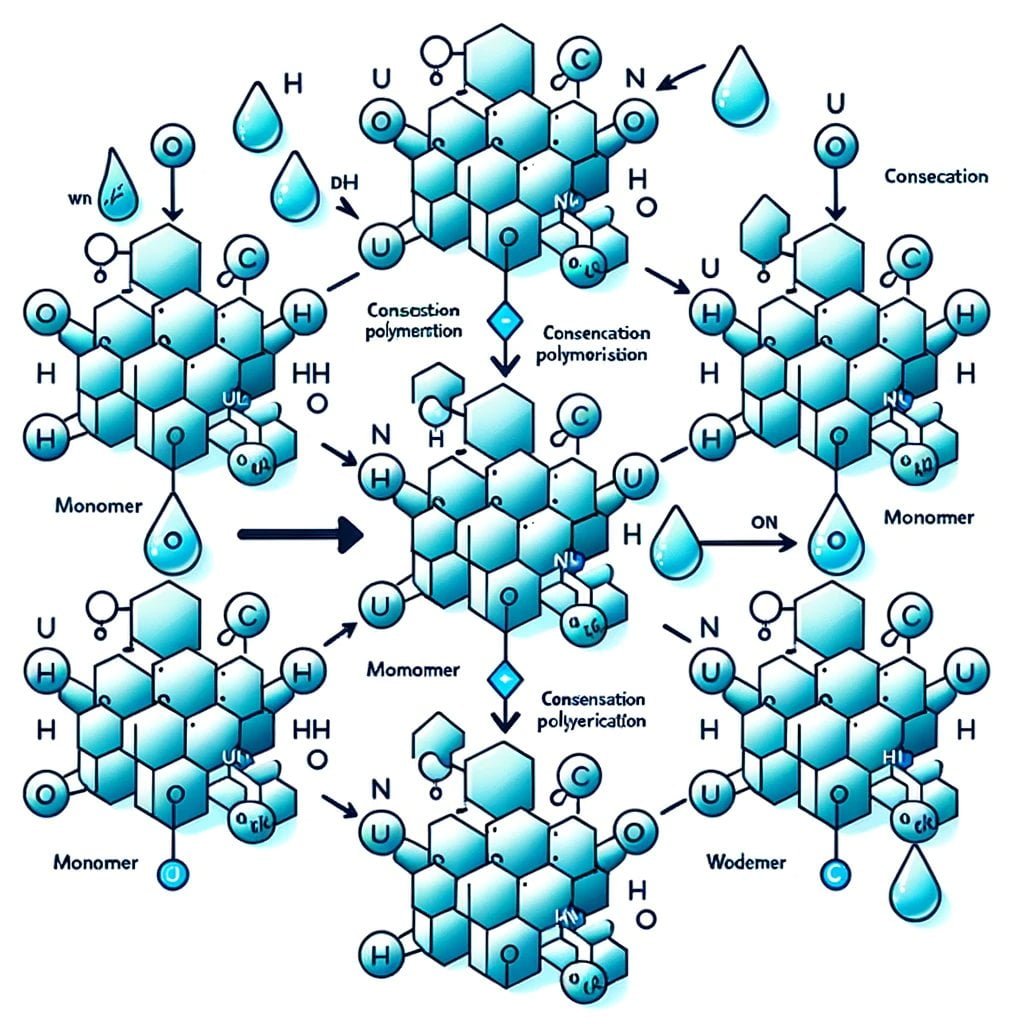
An intriguing phenomenon, condensation polymerization consists of the chemical reaction of oligomers and monomers to form larger structural units. In contrast to the iterative process of addition polymerization, condensation polymerization generates polymers via the removal of small molecules, such as methanol or water, as residues. Condensation polymerization derives its name from this distinctive property, since it involves a succession of condensation reactions.
Monomers and oligomers that are involved in condensation polymerization all have two reactive end groups. These end groups may consist of, among other things, alcohol, amine, or carboxylic acid. The aesthetic appeal of this procedure resides in its capacity to amalgamate functional groups or monomers that are either similar or dissimilar, thereby generating an extensive array of polymers characterized by their varied properties and applications.
Condensation polymerization is distinguished by the necessity for monomers to possess two functional groups. The presence of these functional groups facilitates the monomers’ association with two additional monomers, thereby producing linear polymers. Nylon and polyester are condensation polymers that find extensive application in various sectors, including textiles, automotive, and packaging.
The rate of condensation polymerization is comparatively sluggish in contrast to addition polymerization due to the generation of small molecules as residues during the chain formation process. The gradual rate of the reaction further enhances the linear configuration of the polymers generated via this methodology.
As an additional illustration of condensation polymerization, consider the following conundrum: Consider every monomer and oligomer to be a component of a puzzle, with two reactive end groups serving as connecting elements. A larger puzzle piece is produced when two pieces with corresponding connectors are assembled, resulting in the release of a smaller piece as a byproduct. As an additional link is inserted, the enigma expands until it presents itself as a linear polymer endowed with distinctive characteristics.
To summarize the fundamentals of condensation polymerization:
By converting monomers and/or oligomers into larger structural units via condensation polymerization, small molecules are liberated as byproducts.
Condensation polymerization involves monomers and oligomers that each contain two reactive end groups.
By means of polymerization, linear polymers are generated.
Nylon and polyester are two instances of condensation polymers.
Condensation polymers require two functional groups per monomer in order to form bonds with two additional monomers.
Consumption polymerizations are characteristic of monomers that comprise two or more atomic groups that are reactive.
Condensation polymerization, to conclude, is an intriguing procedure that permits the formation of numerous polymers with distinct properties and applications. Engineers and scientists can engineer and optimize polymerization processes to attain desirable results by acquiring knowledge of the fundamental condensation polymerization facts and the mechanisms that underlie them. Therefore, let us further explore the realm of condensation polymerization in order to harness its capacity for advancement and novelty.
“Condensation polymerization brings together puzzle pieces, forming a picture-perfect polymer chain, one connection at a time.”
Condensation polymerization is a fascinating process that results in the creation of large molecules by the elimination of small molecules, such as water or alcohol. If you’re interested in learning more about the intriguing facts surrounding condensation polymerization, you won’t want to miss out on our comprehensive guide. Click here to explore the mesmerizing world of condensation polymerization: facts about condensation polymerization.
FAQ
Condensation polymerization is defined as…
A type of step-growth polymerization, condensation polymerization involves the reaction of monomers and/or oligomers to produce larger structural units. It results in the emission of smaller molecules as metabolites, including water and methanol.
In terms of characteristics, condensation polymerization stands out.
In order to undergo condensation polymerization, compounds must contain a minimum of two functional groups, including carboxylic acid, alcohol, or amine groups. Linear polymers may be the product of a reaction between functional groups or monomers that are similar or dissimilar. For condensation polymers to form bonds with two other monomers, their monomers must contain two functional groups.
Could you provide some instances of condensation polymers?
Polyester and nylon are prevalent instances of condensation polymers. These materials find extensive applicability in a multitude of fields owing to their advantageous characteristics.
In what ways does condensation polymerization deviate from alternative polymerization processes?
In contrast to alternative polymerization processes, including addition polymerization, condensation polymerization generates small molecules as byproducts. With the exception of addition polymerization, these byproducts are not produced.
Why is knowledge of condensation polymerization crucial for a chemical engineer to possess?
For the purpose of designing and refining polymerization processes, knowledge of condensation polymerization is vital for a chemical engineer with a background in polymer science. By offering knowledge on reaction kinetics, thermodynamics, and the formation of particular polymer structures, it empowers engineers to devise inventive and effective resolutions within their respective domains.