# The Ultimate Guide to Chemistry Reference Tables
Chemistry reference tables are essential tools for students, educators, and professionals in the field. These tables compile crucial data, formulas, constants, and other vital information needed to solve problems, understand concepts, and conduct experiments. This comprehensive guide explores the significance, contents, and effective use of chemistry reference tables. For a handy chart of common polyatomic ions, check out this helpful resource: [Polyatomic Ion Chart](https://www.lolaapp.com/chart-of-polyatomic-ions/).
## Why Chemistry Reference Tables Matter
Chemistry is a quantitative science. It relies on precise measurements, established constants, and universally accepted formulas. Memorizing every single piece of information is impractical. Chemistry reference tables provide a readily accessible compilation of this information, allowing users to:
* **Solve Problems Efficiently:** Quickly access needed values and formulas to solve complex chemical problems.
* **Understand Concepts:** Reinforce understanding by connecting abstract concepts to concrete data.
* **Perform Experiments:** Ensure accuracy and consistency in experimental procedures by using standard values for constants and properties.
* **Prepare for Exams:** Master key concepts and problem-solving skills necessary for success in chemistry courses and standardized tests.
## What's Typically Included in Chemistry Reference Tables?
A comprehensive chemistry reference table typically includes the following sections:
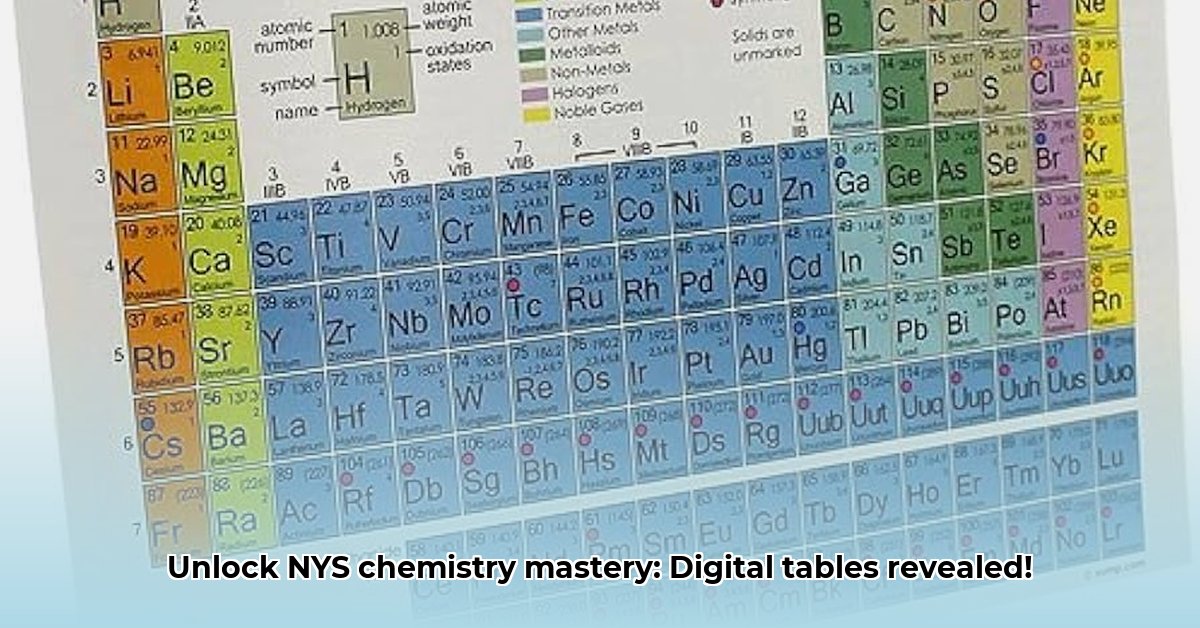
### 1. The Periodic Table of Elements
The periodic table is arguably the most important tool in chemistry. It organizes elements based on their atomic number, electron configuration, and recurring chemical properties. Reference tables usually include a detailed periodic table with the following information for each element:
* **Atomic Number:** Number of protons in the nucleus.
* **Symbol:** Abbreviation for the element's name.
* **Name:** Element's name.
* **Atomic Mass:** Average mass of an atom of the element.
* **Electron Configuration:** Arrangement of electrons in different energy levels and sublevels.
* **Electronegativity:** Measure of an atom's ability to attract electrons in a chemical bond.
* **Ionization Energy:** Energy required to remove an electron from an atom.
* **Common Oxidation States:** Possible charges an atom can have when forming chemical bonds.
### 2. Physical Constants
Physical constants are fundamental quantities that occur frequently in chemistry calculations. Some common physical constants include:
* **Avogadro's Number (N<sub>A</sub>):** 6.022 x 10<sup>23</sup> mol<sup>-1</sup> (Number of entities in one mole).
* **Gas Constant (R):** 8.314 J/(mol·K) or 0.0821 L·atm/(mol·K) (Relates energy, temperature, and amount of substance for gases).
* **Faraday Constant (F):** 96485 C/mol (Magnitude of electric charge per mole of electrons).
* **Speed of Light (c):** 2.998 x 10<sup>8</sup> m/s (Speed at which light travels in a vacuum).
* **Planck's Constant (h):** 6.626 x 10<sup>-34</sup> J·s (Relates energy to frequency of electromagnetic radiation).
### 3. Properties of Water
Water is essential to chemistry, and its properties are frequently used. Reference tables often include:
* **Density:** Mass per unit volume (approximately 1 g/mL at room temperature).
* **Specific Heat Capacity:** Energy required to raise the temperature of 1 gram of water by 1 degree Celsius (4.184 J/g·°C).
* **Boiling Point:** Temperature at which water changes from liquid to gas (100 °C at standard pressure).
* **Freezing Point:** Temperature at which water changes from liquid to solid (0 °C at standard pressure).
* **Heat of Fusion:** Energy required to melt 1 gram of ice (334 J/g).
* **Heat of Vaporization:** Energy required to vaporize 1 gram of water (2260 J/g).
* **Ion Product Constant (K<sub>w</sub>):** [H<sup>+</sup>][OH<sup>-</sup>] = 1.0 x 10<sup>-14</sup> at 25 °C (Equilibrium constant for the autoionization of water).
### 4. Thermodynamic Data
Thermodynamic data relates to energy changes in chemical reactions. Common entries include:
* **Standard Enthalpy of Formation (ΔH<sub>f</sub>°):** Change in enthalpy when one mole of a compound is formed from its elements in their standard states.
* **Standard Gibbs Free Energy of Formation (ΔG<sub>f</sub>°):** Change in Gibbs free energy when one mole of a compound is formed from its elements in their standard states.
* **Standard Entropy (S°):** Measure of disorder or randomness of a substance at standard conditions.
### 5. Solubility Rules
Solubility rules provide guidelines for predicting whether a compound will dissolve in water. These rules are essential for precipitation reactions and understanding aqueous solutions.
* **General Rules:** Listing of common ions that are usually soluble or insoluble.
* **Exceptions:** Listing of exceptions to the general rules.
### 6. Electrochemical Data
Electrochemical data is used in electrochemistry, which studies the relationship between chemical reactions and electrical energy.
* **Standard Reduction Potentials (E°):** Measure of the tendency of a chemical species to be reduced.
* **Activity Series:** Listing of metals in order of their reactivity.
### 7. Formulas and Equations
Reference tables contain important formulas and equations from various areas of chemistry:
* **Stoichiometry:** Mole concept, molar mass, percent composition.
* **Gas Laws:** Ideal gas law, Boyle's law, Charles's law, Avogadro's law.
* **Thermochemistry:** Heat capacity, enthalpy change, Hess's law.
* **Chemical Kinetics:** Rate laws, Arrhenius equation.
* **Equilibrium:** Equilibrium constant, Le Chatelier's principle.
* **Acids and Bases:** pH, pOH, acid-base titrations, buffer solutions.
* **Electrochemistry:** Nernst equation, Faraday's laws of electrolysis.
### 8. Common Ions and Polyatomic Ions
* **Cations:** Positively charged ions (e.g., Na<sup>+</sup>, Ca<sup>2+</sup>, Al<sup>3+</sup>).
* **Anions:** Negatively charged ions (e.g., Cl<sup>-</sup>, O<sup>2-</sup>, N<sup>3-</sup>).
* **Polyatomic Ions:** Ions composed of multiple atoms (e.g., SO<sub>4</sub><sup>2-</sup>, NO<sub>3</sub><sup>-</sup>, NH<sub>4</sub><sup>+</sup>).
### 9. Organic Chemistry Functional Groups
For organic chemistry, reference tables include a list of common functional groups:
* **Alkanes, Alkenes, Alkynes:** Basic hydrocarbon structures.
* **Alcohols:** Contain -OH group.
* **Ethers:** Contain -O- group.
* **Aldehydes:** Contain -CHO group.
* **Ketones:** Contain -CO- group.
* **Carboxylic Acids:** Contain -COOH group.
* **Esters:** Contain -COOR group.
* **Amines:** Contain -NH<sub>2</sub>, -NHR, or -NR<sub>2</sub> group.
* **Amides:** Contain -CONH<sub>2</sub> group.
### 10. Nuclear Chemistry Symbols
Reference tables also contain symbols used in nuclear chemistry.
* **Alpha Particle:** <sup>4</sup><sub>2</sub>He
* **Beta Particle:** <sup>0</sup><sub>-1</sub>e
* **Gamma Ray:** γ
* **Neutron:** <sup>1</sup><sub>0</sub>n
* **Proton:** <sup>1</sup><sub>1</sub>H
## How to Effectively Use Chemistry Reference Tables
* **Familiarize Yourself:** Take the time to understand the organization and contents of the reference tables.
* **Practice Retrieval:** Practice locating information quickly and efficiently.
* **Understand Units:** Pay close attention to units and ensure consistency in calculations.
* **Apply Correctly:** Use the correct formulas and equations for the specific problem or situation.
* **Cross-Reference:** Utilize different sections of the reference tables to confirm information and gain a more complete understanding.
## Conclusion
Chemistry reference tables are indispensable tools for anyone working in chemistry. By understanding their contents and effectively using them, students, educators, and professionals can solve problems, understand concepts, and conduct experiments with greater accuracy and efficiency. They provide a foundation upon which deeper understanding and mastery of chemistry can be built.
Latest posts by Lola Sofia (see all)